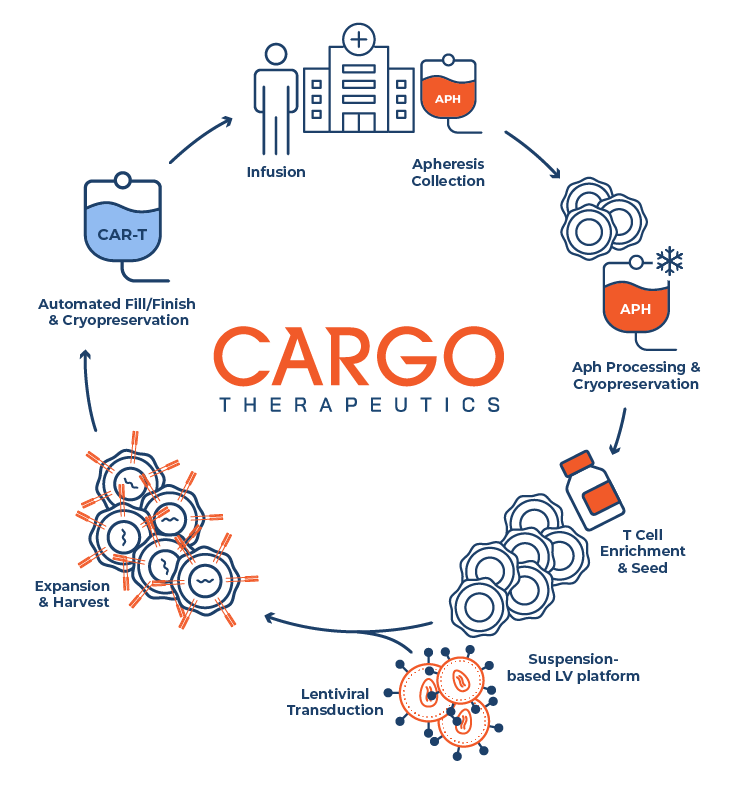
At CARGO, we believe reliable and predictable supply remains a challenge for existing CAR T-cell therapies. To address these challenges, we have developed a potentially commercially suitable manufacturing process that uses an automated and closed platform that is designed to be readily transferrable to multiple manufacturing facilities.
Robust Manufacturing Processes
Our team is applying its extensive experience in the field in an effort to implement manufacturing processes that are highly reliable and readily transferrable to expand capacity, reduce turnaround time and minimize costs of goods. We have also licensed and further developed technologies specifically designed towards the manufacturing and purification of CAR T-cells containing multiple genetic inserts delivered by multiple vectors.