| Allogeneic Platform | Applications | Indication(s) | Discovery | IND-enabling | Phase 1 | Phase 2 | Phase 3 | Commercial rights |
|---|---|---|---|---|---|---|---|---|
| Universal Vector | CAR T-cell therapy | Potential for hem onc, solid tumors, other |  | |||||
| Universal Vector | |||||
|---|---|---|---|---|---|
| Discovery | IND-enabling | Phase 1 | Phase 2 | Phase 3 | Commercial rights |
| Target: CAR T-cell therapy | |||||
| Indication: Potential for hem onc, solid tumors, other | |||||
Universal allogeneic-enabling vector solution engineered to limit rejection and enable conversion of autologous CAR T-cell therapy for broader patient benefit.
CARGO’s differentiated approach:
At CARGO, we aim to solve the challenges that limit a broader impact for cell therapy, including quality of T cells derived from sick patients and availability of product for autologous cell therapy and durable efficacy for allogeneic cell therapy.
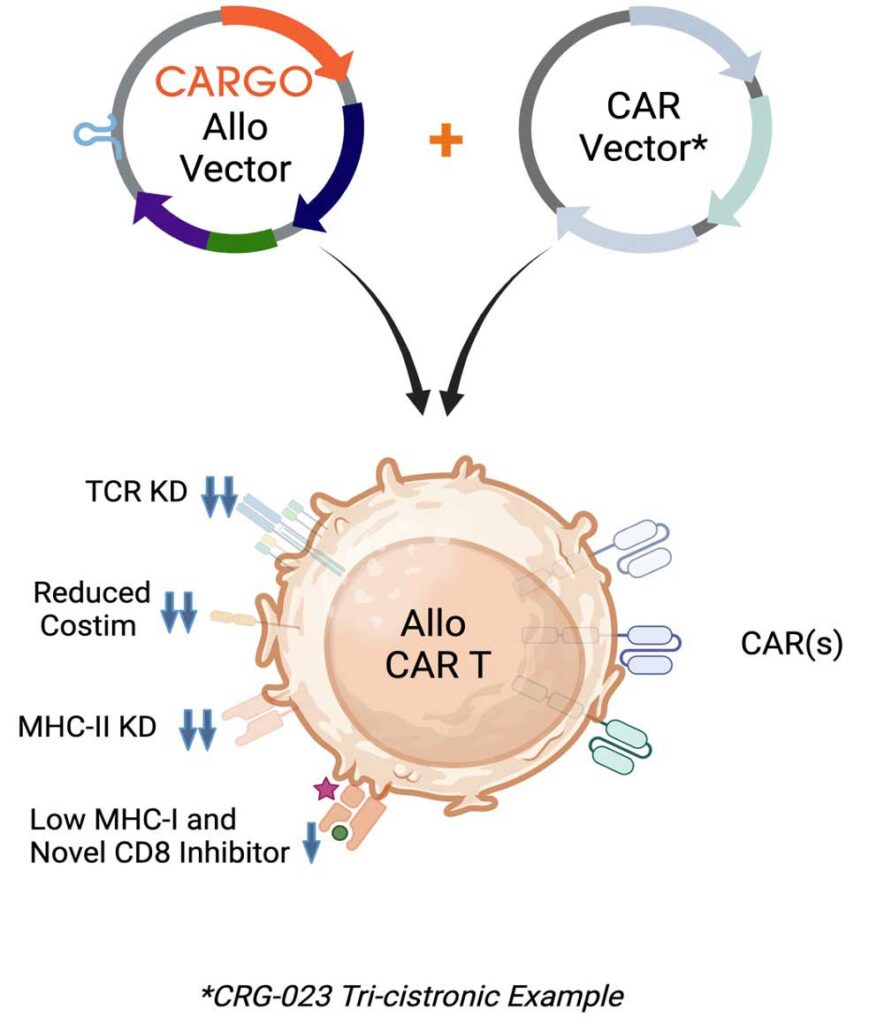
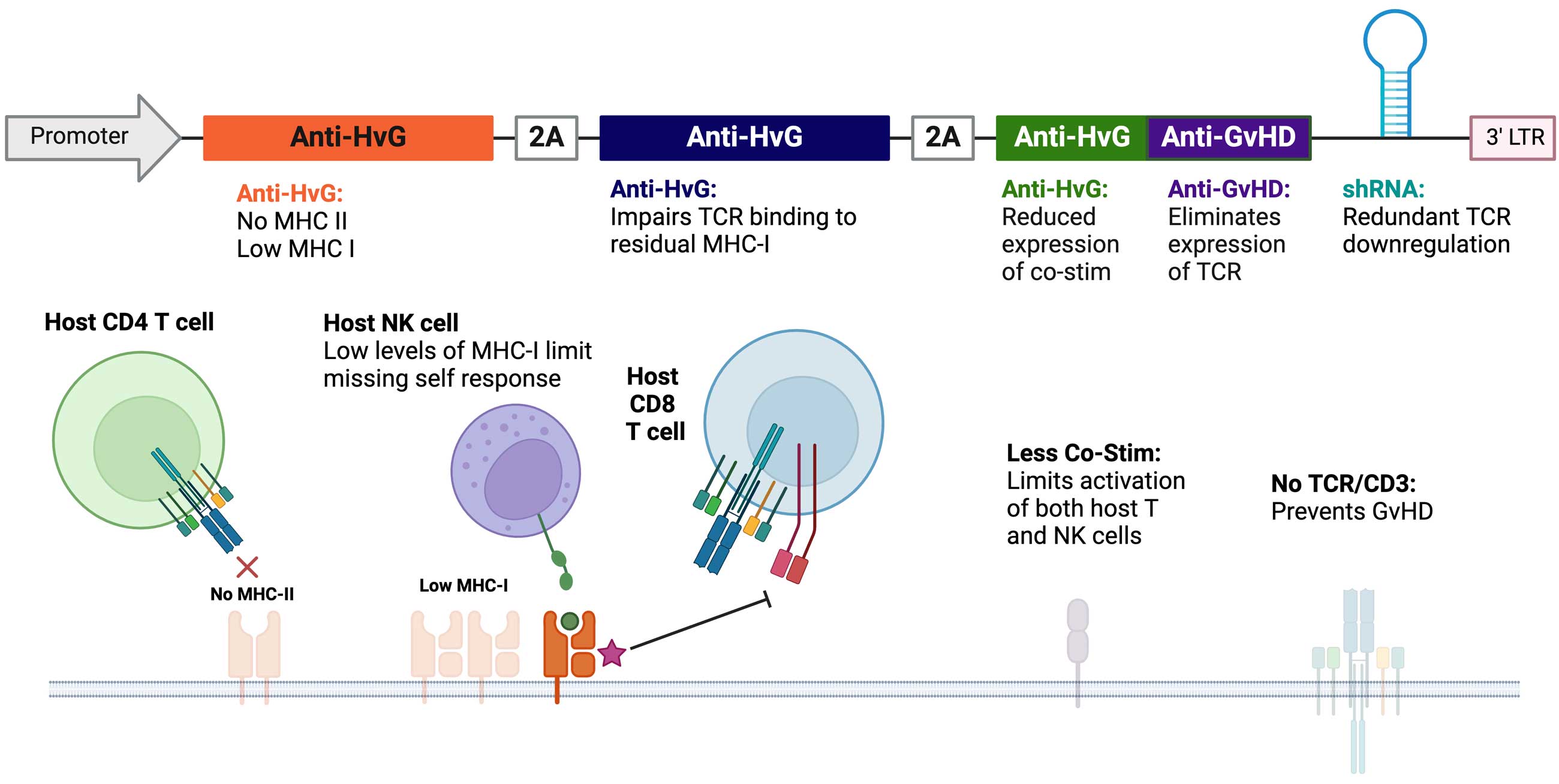
Through sophisticated cell therapy design and engineering, CARGO’s novel allogeneic platform is a universal vector solution designed to limit immune-based rejection and enable durable response of CAR T-cell therapy.
Our differentiated platform is intended to transform potentially curative autologous CAR T-cell therapies into allogeneic products for broader patient benefit. The universal vector is engineered with multiple transgene “cargo” to limit T and natural killer (NK) cell rejection and downregulate T cell receptor (TCR). Importantly, the vector is intended to enable pairing with new or clinically established CAR vectors to create an allogeneic CAR T-cell therapy product. Further, this pairing can be accomplished while leveraging existing autologous drug product processes.
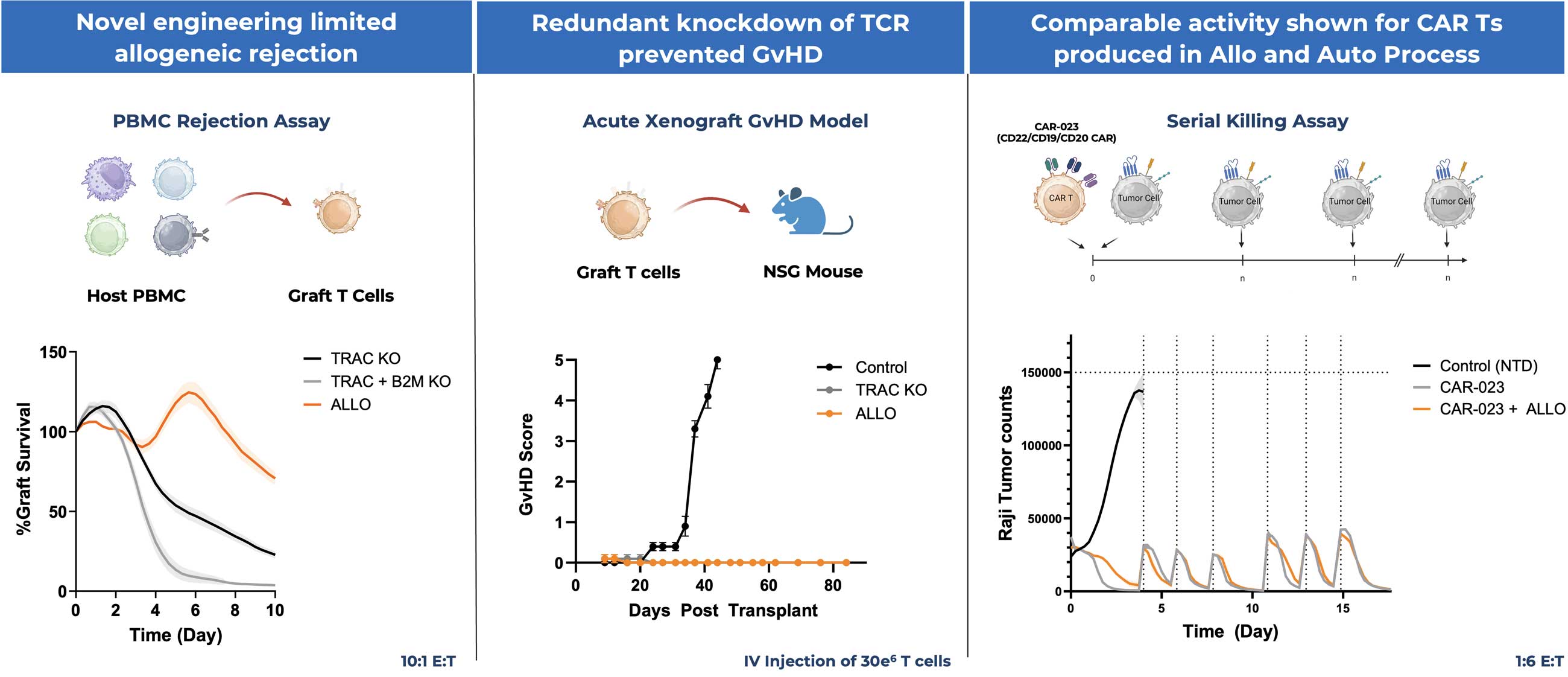
The resulting off-the-shelf allogeneic CAR T product is intended to limit rejection, promote safety, improve the starting quality of T cells, and maintain comparable CAR-mediated activity.
Meaningful Development Progress
- Advanced lead vectors with novel engineering to limit immune rejection
- Safety demonstrated in vivo – prevention of GvHD without gene editing
- Preserved CAR activity with co-transduced, GMP CAR vector
- Demonstrated feasibility of co-transduction to produce allogenic CAR T cells with high purity and multiple doses at meaningful manufacturing scale
Preclinical Data Demonstrate Proof of Concept Across Assessments