| Autologous Program | Target(s) | Indication(s) | Discovery | IND-enabling | Phase 1 | Phase 2 | Phase 3 | Commercial rights |
|---|---|---|---|---|---|---|---|---|
| CRG-023 (tri-specific, tri-cistronic CAR T) | CD19 CD20 CD22 |
B-cell malignancies |  | |||||
| CRG-023 (tri-specific, tri-cistronic CAR T) | |||||
|---|---|---|---|---|---|
| Discovery | IND-enabling | Phase 1 | Phase 2 | Phase 3 | Commercial rights |
| Target: CD19 CD20 CD22 | |||||
| Indication: B-cell malignancies | |||||
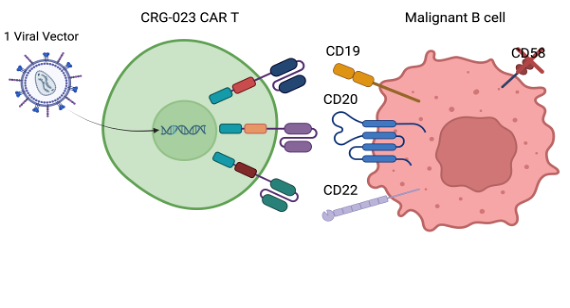
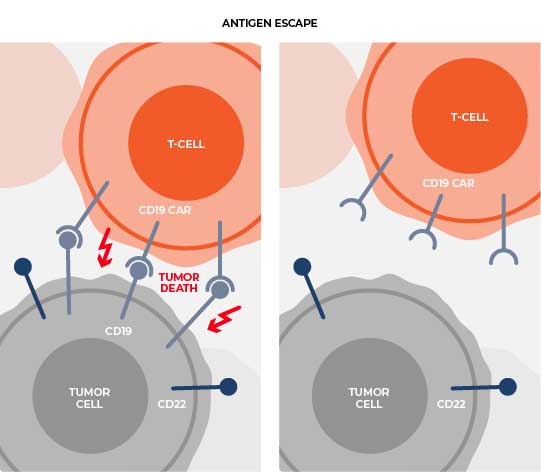
CRG-023 is a CD19, CD20, CD22 targeting tri-specific CAR T product candidate designed with the goal of providing more patients with a broad range of B-cell malignancies with durable responses by addressing several known causes of relapse, resulting in a potential best-in-class CAR T-cell therapy. Causes of relapse associated with existing CAR T-cell therapies include tumor antigen loss (e.g., CD19), loss of co-stimulation (e.g., CD58) and T cell exhaustion.
We are Unique in Our Approach to Optimizing Many Aspects of Cell Therapy for Patient Benefit
Design Matters
CRG-023 is the first of its kind, tri-cistronic CAR T to express three independent CARs from a single vector, with each CAR having a distinct co-stimulatory domain. One co-stimulatory domain incorporates CD2 signaling into the tri-specific CAR T cell, the design of which has been informed by immune-evasion and CAR T cell resistance mediated by the loss of CD58 (the ligand of the CD2 co-stimulatory receptor).
Novel CD19 and CD20 scFv binders were selected for enhanced CAR performance and combined with the existing CD22 scFv binder used in firicabtagene autoleucel (firi-cel). These new binders are fully human to limit host-mediated rejection. The iterative engineering of the construct allowed for the selection of the optimal costimulatory domain configuration and optimal assemblage in a unique tri-cistronic lentiviral vector to maximize durable anti-tumor response.
Intentional engineering and optimization of the final construct sought to limit onset of functional exhaustion and to sustain performance across a range of antigen expression profiles to prevent antigen escape.
Through this innovative design and sophisticated engineering, CARGO aims to deliver multiple, therapeutically beneficial transgene “cargo” from a single vector.
CAR CD2 Costimulatory Domain
The strength and quality of a T-cell response is dependent not only on cognate antigen recognition, but also on costimulation, which involves interaction of one or more costimulatory receptors on T cells, such as CD2, with ligands expressed on the surface of tumor cells. Tumor cells can escape CAR T-cell destruction by downregulating the expression of ligands for the costimulatory receptors. These ligands include CD58, the ligand of the CD2 costimulatory receptor. Alteration of CD58 expression is associated with poor prognosis in LBCL and leads to lack of response to CD19 CAR T cells. Informed by these observations, we incorporated a CD2 costimulatory domain on to the CD20 CAR. This design feature results in impressive durable, CAR-mediated activation.
Preclinical Data Highlights
CRG-023 Abstract – 2024 American Society of Hematology (ASH) Annual Meeting
- Sustained anti-tumor activity and a lack of functional exhaustion during repeated challenge from tumor cells.
- Preservation of T cell memory phenotype relative to controls, sustained tumor clearance when only a single antigen is expressed.
- Robust and sustained in vivo, anti-lymphoma activity at low CAR T dose levels.