Our sophisticated design and engineering capabilities underpin our programs and platform technologies developed to address the current limitations of cell therapy. Challenges that limit a broader impact for autologous cell therapy include mechanisms of resistance, the quality of T cells derived from sick patients and availability of product. For allogeneic cell therapy, the primary challenge remains helping patients achieve durable efficacy.

CARGO’s Approach
CARGO strives to optimize all components of a CAR to unlock therapeutic potential for patients. Our solution includes:
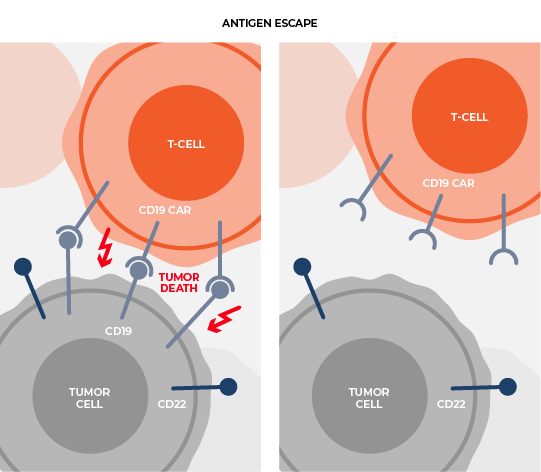
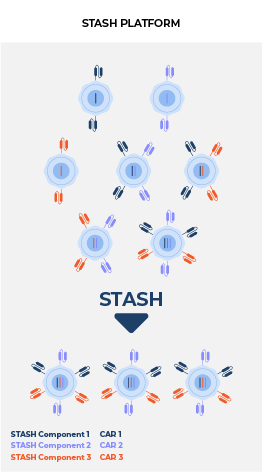
Delivering multiple, therapeutically beneficial transgene “cargo” from a single vector. Therapies that target single tumor antigens, such as CD19, can be rendered ineffective by genetic or non-genetic changes that diminish the expression of these targets. Our lead product candidate CRG-023, is a tri-specific CAR T targeting three B-cell lineage antigens (CD19, CD20, and CD22) via three separately expressed CARs on a single vector. The construct enables the recognition of tumors that express any one of the CD19, CD20, and CD22 antigens, thereby limiting potential antigen loss as a mechanism of resistance. Thousands of constructs were screened to identify a vector configuration that enabled effective CAR expression and anti-tumor activity. Finally, intentional engineering and optimization of the final construct was completed to limit onset of functional T-cell exhaustion and to sustain performance across a range of antigen expression profiles in order to prevent antigen escape.
Addressing common mechanism of non-target-based resistance. In addition to antigen downregulation or loss, resistance to immune therapies, including CAR T cells, can develop through the loss of costimulatory signaling, such as tumor cells downregulating CD58 expression. Because these mechanisms are not antigen- specific, loss of costimulation can lead to broad suppression of immune therapies. We are working to address loss of costimulatory ligands such as CD58, by creating CAR T cells that can induce CD2 costimulatory signaling by a tumor antigen irrespective of potential CD58 downregulation or loss on tumor cells. For CRG-023, we designed each of the CD19, CD20, and CD22 CARs to have a distinct costimulatory domain. The CD20 CAR design incorporates a CD2 costimulatory domain.
Using fully-human binders to reduce anti-CAR immunogenicity. CRG-023 is constructed with only human binders, thereby reducing the risk for anti-CAR immune responses. CARGO implemented an extensive new human binder campaign for CD19 and CD20 and selected binders in CAR format based on optimal activity.
Broadening the availability of safe and effective cell therapy. CARGO’s novel allogeneic platform is a universal vector solution designed to limit immune-based rejection of allogeneic CAR T and enable durable response. The allogeneic CAR T platform is comprised of a universal vector with multiple transgene “cargo” to limit T and NK rejection and downregulate TCR. Importantly, the universal, allogeneic-enabling vector is intended to pair with new or clinically established CAR vectors, while leveraging existing autologous drug product processes. The result is intended to be an off-the-shelf allogeneic CAR T product designed to limit rejection, promote safety, improve the starting quality of T cells, and maintain comparable CAR-mediated activity.
Through sophisticated engineering, the differentiated platform aims to transform potentially curative autologous CAR T-cell therapies into allogeneic products for broader patient benefit.